Welcome to the Medical Device Made Easy podcast. Here is Mineral Azusi from idiomedicaldevice.com, and today we will talk about a tsunami, earthquake, or something that happened within the medical device industry. We'll discuss this with Eric Verbrick. So, Eric, welcome to the Medical Device Made Easy podcast. Hi everyone! So, Eric, I mean, December is a month of normally some kind of gifts with some Christmas presents, etc. So we had some announcements. But I will ask you, maybe, is it a Christmas present or not? We had a situation that was happening on December 9th at the EPS Committee in Brazil. Can we just put a bit of chronology in place of this for sure things, and then we try to comment on it? Basically, I would say where it all started was with the MDCG 2020-11 position paper this summer. In this paper, the MDCG started hinting that they might come up with a solution for people with certain expiring certificates, while the notified body is not ready with the Conformity assessment procedure. They had some outlines in the position paper, saying that you should be in manufacturing good standing and have an application in the works at least a year before certificate expiry, among other criteria. However, they didn't explicitly say that this would be article 97 based, as the member states were unsure about how to proceed. There were a lot of companies clamoring for an extension, although it was unclear what it meant exactly. Additionally, France and Germany had been politically mobilized to advocate for the commission to do something about the messy MDR transitional regime. In September, we had the famous MDCG 19 points 2022-14 position paper, which I personally thought was just a wish list, with some definite navies and some suggestions for others to...
Award-winning PDF software





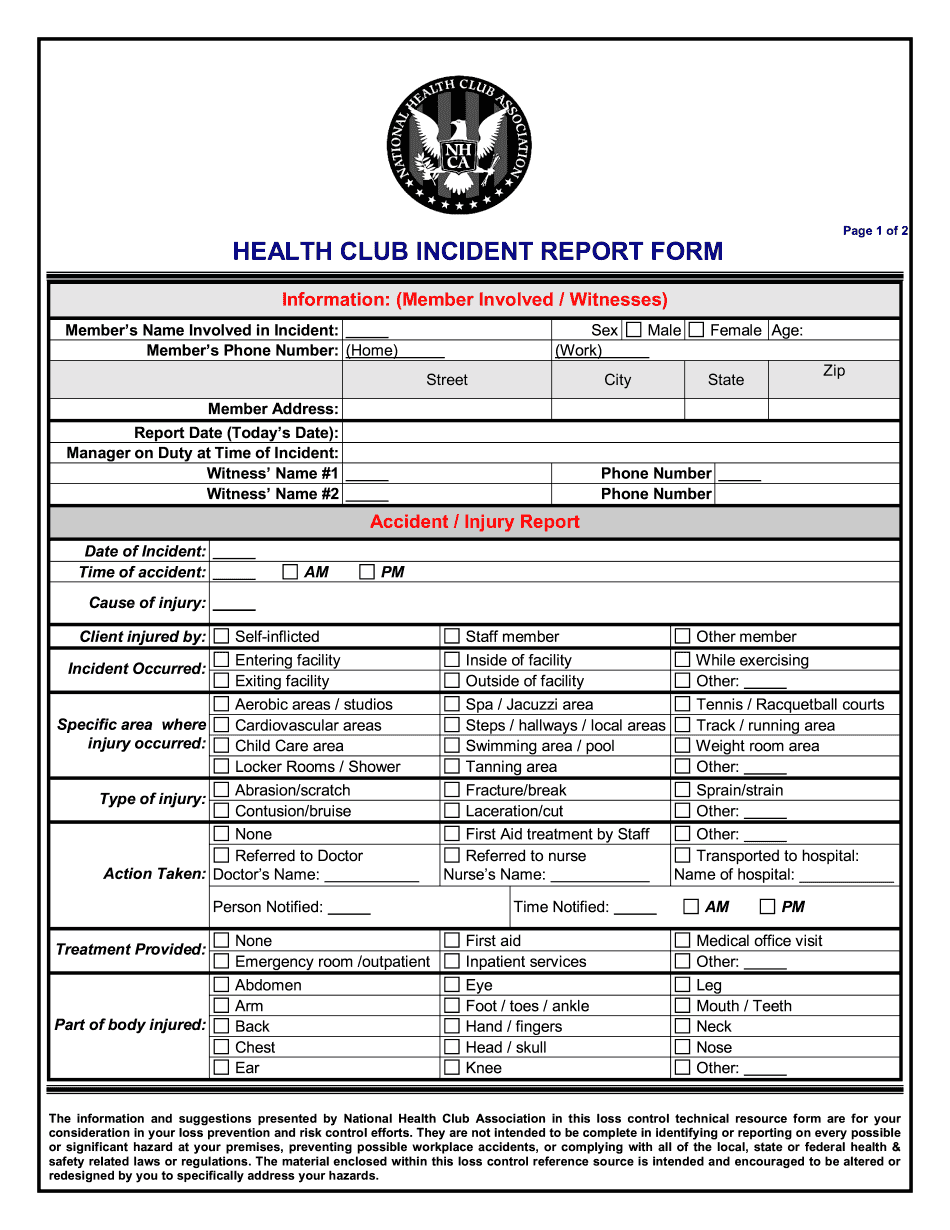
Online choices enable you to to prepare your document management and improve the efficiency of one's workflow. Go along with the quick manual in an effort to entire Health Club incident Report, stay clear of errors and furnish it in a well timed manner:
How to finish a Health Club incident Report over the internet:
- On the website with all the type, click Launch Now and pass towards editor.
- Use the clues to fill out the pertinent fields.
- Include your own facts and get in touch with info.
- Make absolutely sure that you just enter right facts and figures in correct fields.
- Carefully check out the content belonging to the sort too as grammar and spelling.
- Refer to support segment if you've got any issues or deal with our Support workforce.
- Put an electronic signature on the Health Club incident Report with all the aid of Indication Device.
- Once the form is concluded, push Completed.
- Distribute the ready type via e mail or fax, print it out or save on your own machine.
PDF editor lets you to definitely make variations in your Health Club incident Report from any web connected equipment, customise it according to your preferences, indicator it electronically and distribute in various strategies.
Video instructions and help with filling out and completing Health Club incident Report