Welcome back to the GCP Mindset channel. As you may know from our previous videos, the essential documents of each clinical study are the study protocol, the investigator's brochure, and the case report form. Today, we talk about the electronic case report form (eCRF). Originally, all case report forms were made on paper, but recently there is a changing trend to clinical studies using an electronic case report form. The research-based industry has increasingly begun to use these eCRFs. The associated process of electronic acquisition of data at the trial site is referred to as remote data entry or electronic data capture. The use of paper CRF causes the creation of numerous queries by the data management due to illegible data. By using eCRFs, the bad handwriting of the person filling in the eCRF is not applicable as an error source because the legibility of the data is ensured at all times. Thus, fewer queries are created. The reduced paper consumption should also be seen from economic and ecological viewpoints. Quicker data transmission is possible because paper files no longer have to be transported. Monitoring can be planned and prepared by the monitor based on a review of data from a distance. A direct data check for plausibility is possible, as well as work operations such as SAE reporting, which can be considerably facilitated by automated processes. Furthermore, numerous plausibility checks can be programmed in an eCRF so that many incorrect entries, for example entering the current date as the date of birth, are not possible at all. This enables remote monitoring in which the data entered is checked for correctness and completeness, while the focus of monitoring in the study center is on source data verification (SDV), for example the comparison between the database entries and the source documents....
Award-winning PDF software





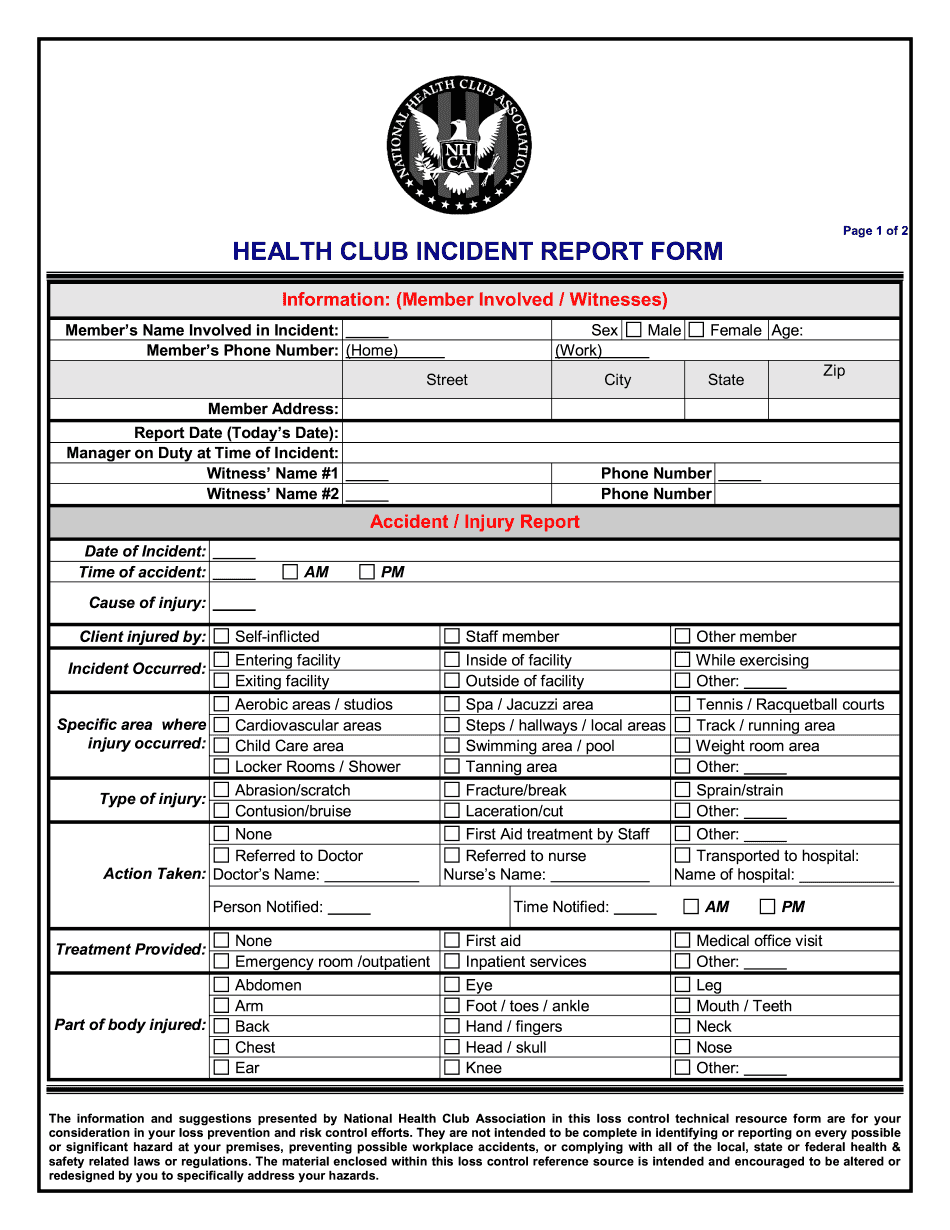
Online systems assist you to to organize your doc management and improve the efficiency of your workflow. Adhere to the fast information to be able to total Health Club incident Report, keep clear of errors and furnish it inside of a timely method:
How to accomplish a Health Club incident Report internet:
- On the website together with the sort, click Start off Now and move with the editor.
- Use the clues to fill out the appropriate fields.
- Include your own material and speak to details.
- Make guaranteed that you just enter suitable data and figures in correct fields.
- Carefully examine the subject material of the form likewise as grammar and spelling.
- Refer that will help segment if you have any questions or address our Aid group.
- Put an electronic signature with your Health Club incident Report while using the aid of Sign Tool.
- Once the shape is finished, push Performed.
- Distribute the completely ready kind by means of e-mail or fax, print it out or help you save in your equipment.
PDF editor helps you to definitely make alterations to the Health Club incident Report from any online linked equipment, customize it based on your requirements, indication it electronically and distribute in various options.
Video instructions and help with filling out and completing Health Club incident Report